-
Posts
66,900 -
Joined
Content Type
Profiles
Blogs
Forums
American Weather
Media Demo
Store
Gallery
Everything posted by dendrite
-
-
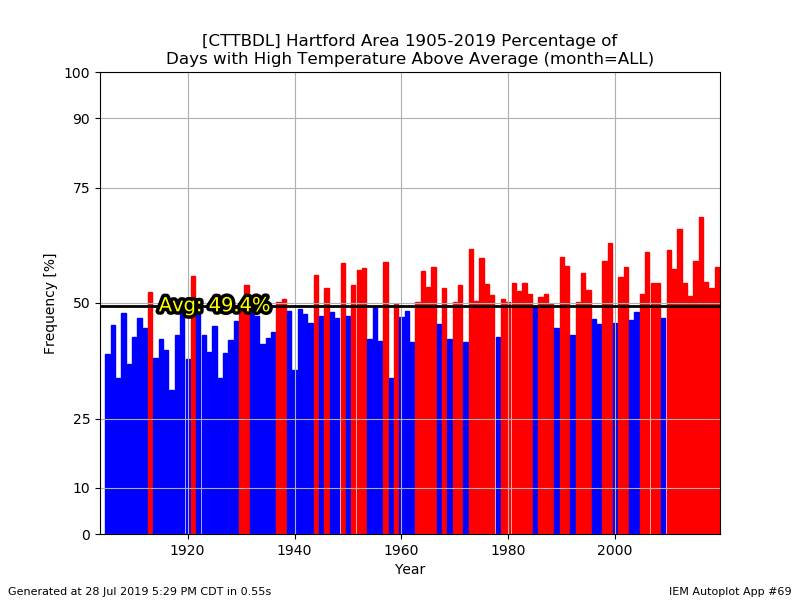
BOS +4.7 BDL +4.1 PWM +3.3 CON +3.1 ORH +3.0 PVD +2.5
-
So yeah...found a bee nest on the edge of my lawn in the backyard. They look pretty active flying in and out of the hole in the ground. I like me some bees so I don't want to kill them. What do we have for ground nesting bees around here? They almost looked like honeybees, but I didn't get a great look since it was 8pm. Is there a humane way to get them to set up shop elsewhere?
-
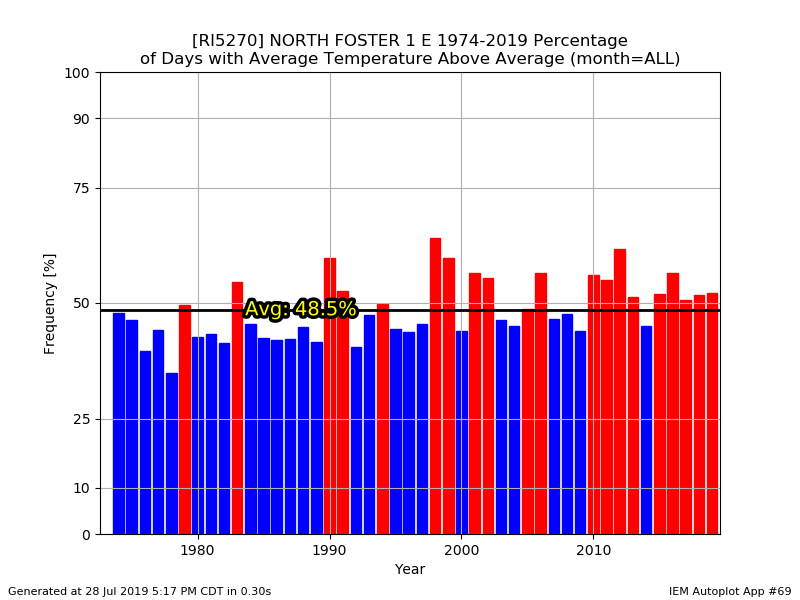
What was the avg high at 945ft?
-
Snoozefest at PVD and ORH STATION: PROVIDENCE RI MONTH: JULY YEAR: 2019 LATITUDE: 41 43 N LONGITUDE: 71 26 W TEMPERATURE IN F: :PCPN: SNOW: WIND :SUNSHINE: SKY :PK WND ================================================================================ 1 2 3 4 5 6A 6B 7 8 9 10 11 12 13 14 15 16 17 18 12Z AVG MX 2MIN DY MAX MIN AVG DEP HDD CDD WTR SNW DPTH SPD SPD DIR MIN PSBL S-S WX SPD DR ================================================================================ 1 84 61 73 1 0 8 0.00 0.0 0 8.6 20 350 M M 3 25 350 2 82 68 75 3 0 10 0.00 0.0 0 7.5 16 250 M M 6 18 240 3 88 66 77 5 0 12 0.00 0.0 0 5.8 13 150 M M 3 16 150 4 90 66 78 5 0 13 0.00 0.0 0 4.8 14 140 M M 5 18 17 150 5 82 66 74 1 0 9 0.00 0.0 0 7.6 15 200 M M 5 1 15 200 6 89 73 81 8 0 16 0.02 0.0 0 9.2 23 230 M M 8 1 27 330 7 80 63 72 -1 0 7 0.00 0.0 0 9.5 20 30 M M 5 26 40 8 81 60 71 -2 0 6 0.00 0.0 0 5.3 12 210 M M 4 13 150 9 88 62 75 2 0 10 0.00 0.0 0 5.7 16 160 M M 5 21 50 10 89 64 77 4 0 12 0.00 0.0 0 6.2 18 150 M M 6 28 160 11 83 69 76 3 0 11 0.06 0.0 0 9.6 22 200 M M 8 18 27 210 12 89 71 80 6 0 15 1.01 0.0 0 4.9 15 30 M M 6 13 19 360 13 86 70 78 4 0 13 0.00 0.0 0 8.9 17 230 M M 4 24 240 14 90 69 80 6 0 15 0.00 0.0 0 8.6 17 290 M M 2 26 310 15 84 66 75 1 0 10 0.00 0.0 0 6.9 16 300 M M 4 20 300 16 87 63 75 1 0 10 0.00 0.0 0 7.2 16 160 M M 6 8 30 110 17 91 72 82 8 0 17 0.39 0.0 0 8.3 21 340 M M 6 138 26 270 18 78 64 71 -3 0 6 0.16 0.0 0 8.1 21 20 M M 8 1 24 20 19 89 65 77 3 0 12 0.00 0.0 0 7.2 16 220 M M 7 21 160 20 94 77 86 12 0 21 0.00 0.0 0 6.9 13 200 M M 5 8 22 210 21 96 78 87 13 0 22 0.00 0.0 0 8.4 20 250 M M 4 28 250 22 84 70 77 3 0 12 0.81 0.0 0 8.5 29 60 M M 6 13 35 60 23 71 64 68 -6 0 3 1.00 0.0 0 8.9 24 10 M M 10 1 47 20 24 82 64 73 -1 0 8 0.02 0.0 0 5.8 14 200 M M 6 1 18 10 25 83 64 74 0 0 9 0.00 0.0 0 5.7 17 200 M M 3 26 170 26 82 63 73 -1 0 8 0.00 0.0 0 6.2 18 150 M M 2 22 150 27 79 64 72 -2 0 7 0.00 0.0 0 7.5 17 150 M M 2 21 140 ================================================================================ SM 2301 1802 0 302 3.47 0.0 197.8 M 139 ================================================================================ AV 85.2 66.7 7.3 FASTST M M 5 MAX(MPH) MISC ----> # 29 60 # 47 20 STATION: WORCESTER MA MONTH: JULY YEAR: 2019 LATITUDE: 42 16 N LONGITUDE: 71 52 W TEMPERATURE IN F: :PCPN: SNOW: WIND :SUNSHINE: SKY :PK WND ================================================================================ 1 2 3 4 5 6A 6B 7 8 9 10 11 12 13 14 15 16 17 18 12Z AVG MX 2MIN DY MAX MIN AVG DEP HDD CDD WTR SNW DPTH SPD SPD DIR MIN PSBL S-S WX SPD DR ================================================================================ 1 80 59 70 1 0 5 0.00 0.0 M 10.4 18 320 M M 2 24 310 2 79 63 71 2 0 6 T 0.0 M 9.6 15 260 M M 2 22 270 3 85 67 76 7 0 11 0.00 0.0 M 4.7 12 310 M M 1 14 320 4 87 67 77 8 0 12 0.00 0.0 M 5.7 10 230 M M 1 15 120 5 84 69 77 7 0 12 0.00 0.0 M 9.7 17 230 M M 1 24 240 6 88 69 79 9 0 14 1.22 0.0 M 10.9 26 300 M M 6 13 36 290 7 79 66 73 3 0 8 0.00 0.0 M 6.5 14 30 M M 5 20 30 8 79 59 69 -1 0 4 0.00 0.0 M 5.4 9 260 M M 0 12 10 9 82 61 72 2 0 7 0.00 0.0 M 6.6 13 340 M M 0 17 320 10 84 64 74 4 0 9 0.00 0.0 M 6.7 12 240 M M 0 14 260 11 79 66 73 3 0 8 0.21 0.0 M 9.2 21 170 M M 5 1 28 160 12 84 68 76 6 0 11 0.72 0.0 M 6.6 17 290 M M 7 12 23 260 13 81 64 73 3 0 8 0.00 0.0 M 7.8 15 290 M M 2 21 270 14 84 67 76 6 0 11 0.00 0.0 M 10.5 17 290 M M 2 21 290 15 78 61 70 -1 0 5 0.00 0.0 M 8.5 17 310 M M 0 23 340 16 82 60 71 0 0 6 T 0.0 M 8.1 15 240 M M 1 20 240 17 86 71 79 8 0 14 0.67 0.0 M 9.7 22 300 M M 4 13 30 320 18 72 63 68 -3 0 3 T 0.0 M 7.5 15 70 M M 9 1 22 50 19 86 64 75 4 0 10 0.00 0.0 M 8.1 17 230 M M 4 22 240 20 90 75 83 12 0 18 0.00 0.0 M 10.4 18 280 M M 1 3 24 280 21 89 73 81 10 0 16 0.00 0.0 M 13.2 24 260 M M 0 32 270 22 78 61 70 -1 0 5 0.82 0.0 M 7.4 15 30 M M 7 13 25 50 23 68 59 64 -7 1 0 1.16 0.0 M 7.2 17 10 M M 8 1 24 10 24 78 62 70 -1 0 5 T 0.0 M 6.0 13 340 M M 3 17 300 25 79 63 71 0 0 6 0.00 0.0 M 4.4 12 300 M M 2 14 310 26 82 62 72 1 0 7 0.00 0.0 M 5.2 14 170 M M 0 17 170 27 80 63 72 1 0 7 0.00 0.0 M 8.7 14 220 M M 3 123 19 210 ================================================================================ SM 2203 1746 1 228 4.80 0.0 214.6 M 76 ================================================================================ AV 81.6 64.7 7.9 FASTST M M 3 MAX(MPH) MISC ----> # 26 300 # 36 290 ================================================================================
-
It's been damn warm down there, but idk...just seems like you're getting there in a boring way. There just hasn't been much in the way of BN days to offset the warmth. I'm still throwing an asterisk at BDL and BOS too.
-
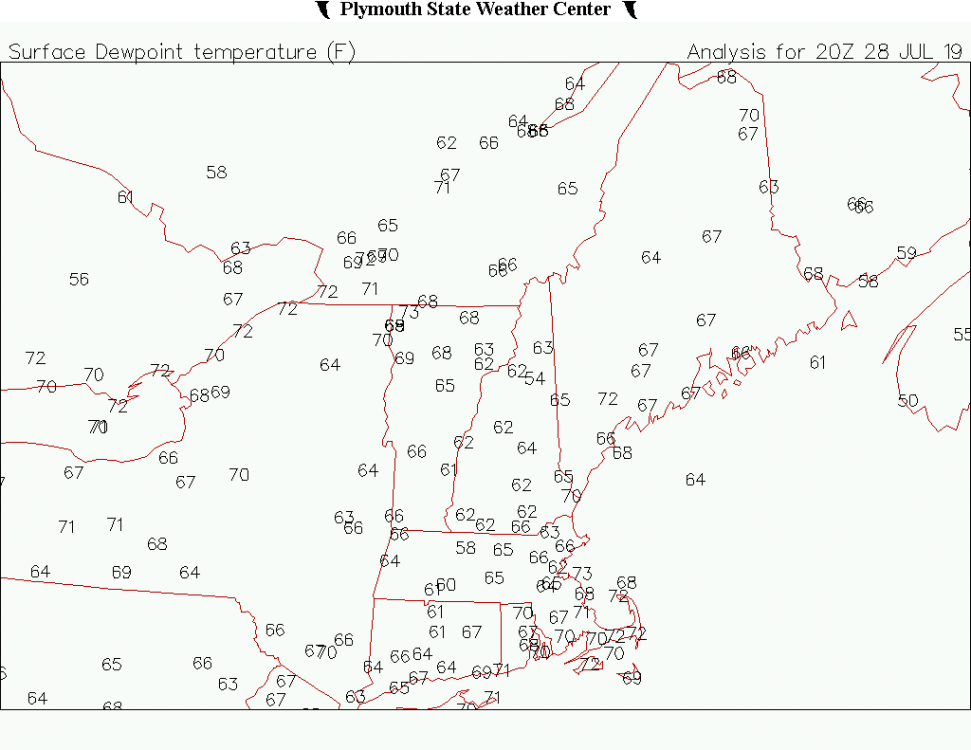
Low 65.5F Not too bad here this morning. Getting a little dewy around the coast, but I think the official sites up here will keep them in the M/U 60s today and tomorrow so naso bad.
-
You had one good heat/dew wave so far. Otherwise it's been typical summer warmth. Unmemorable summer.
-
Yeah I've been saying the trough axis is a little far west for us...think I said +1 to +2 to Kev the other day. GEFS have a decently cool period in the d11-15 period as the axis shifts a bit east. But yeah, looks like typical August doldrums for a bit.
-
Looks like you have it mulched well. I’m not sure it needs daily watering at this point. Stick your finger under the mulch and into the ground. If the top 1-2” is dry maybe give it a deep watering and wait a few days. If it’s moist...just leave it. They’re a shallow rooted plant, but they don’t like to be overwatered either. I had a cherry tree lose all of its leaves last summer after I planted it in the spring, but it put out a little new growth in September and then came back fine this spring. So you’ll probably be fine.
-
I heard a few rumbles from that. Lookin like no dice here.
-
There’s calibration screws on each side. I forget how much of a turn you have to make to equal X hundredths of an inch. But anyway, unscrewing them raises them which in turns means less water is needed to tip the tipper. So that’s the direction you’d want to go to make it read more. What I did was just turn each side the same amount (like a half turn) and keep the stratus gauge near it and wait for a couple of larger rain events to see how much it changed and how much more I needed. Of course make sure the gauge is perfectly level in every direction before making cal screw changes. I think they have a bubble level on them now.
-
I should've stalked your Davis and calibrated it for you while you stalked Eek.
-
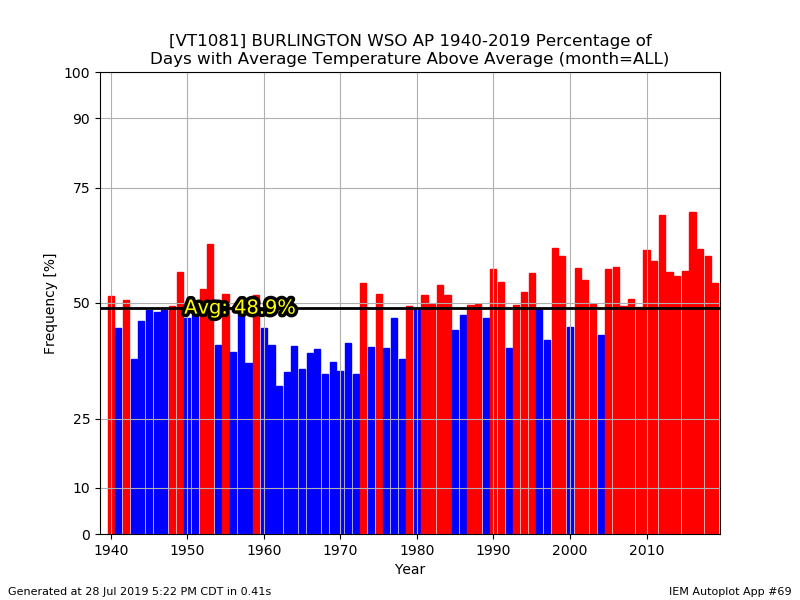
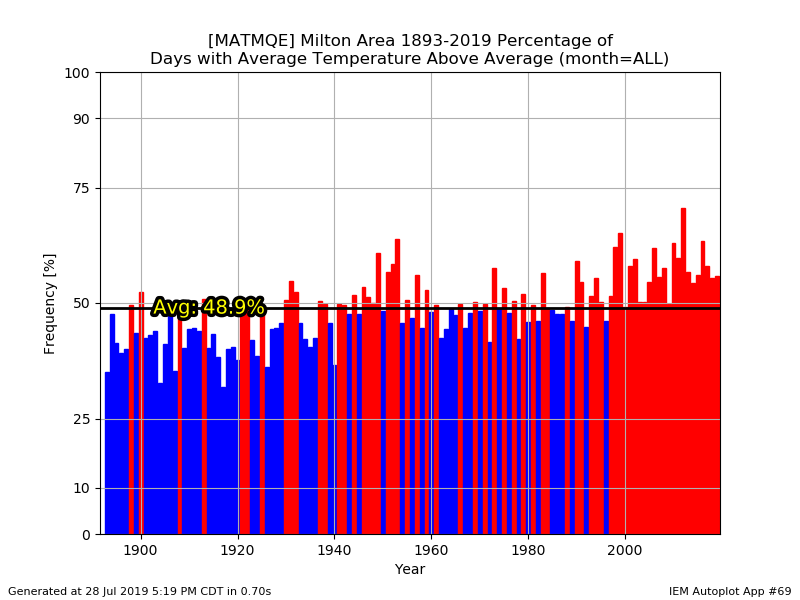
The 1991-2020 normals should be nice and toasty with abundant snow. A bounce back would probably make the 2020s look like the 80s....cold and dry.
-
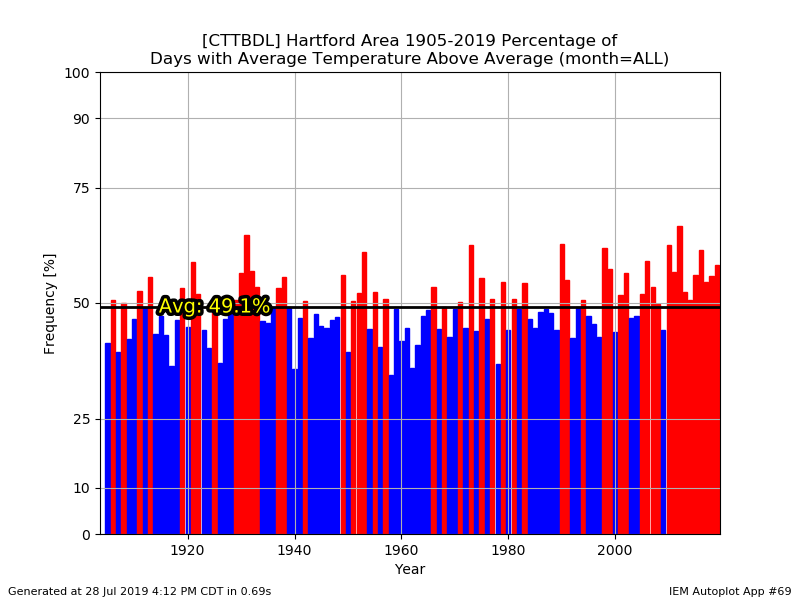
You really can't see the difference there between 1955-2005 and 2005-present?....aka the BDL years.
-
Hey...I wish we could trend colder again, but it is what it is. I agree that the mins are worse though. We're kinda beating a dead horse though. We all know we've been warming for decades.
-
lol...yeah. Just a little bit of a site change for ORH in the 1940s. 7/4/1911 ORH has 102F and BOS 104F. The BOS 103F in 2011 got ORH a 97F.
-
-
-
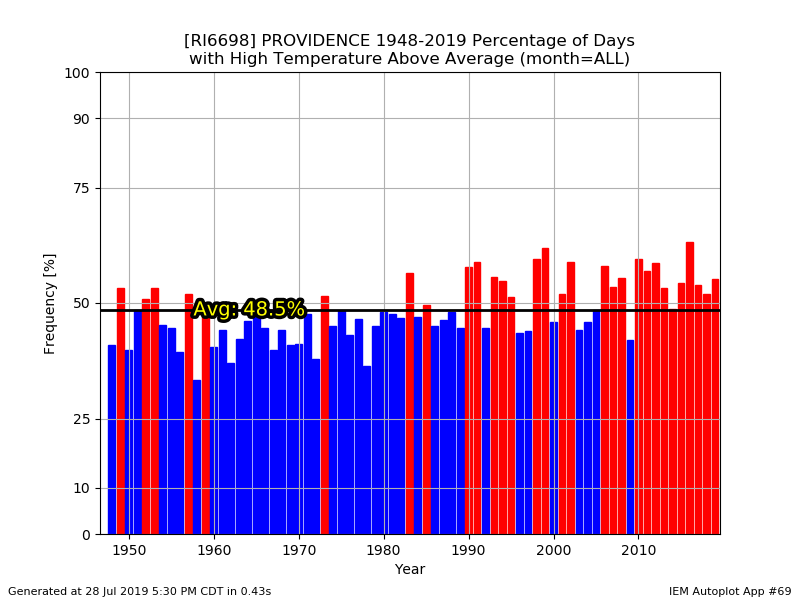
They didn't have just Windsor Locks as an option. And it doesn't change my point anyway. Feel free to eyeball the data since 1948...it's still been a torchfest since the late 90s.
-
-
If you want legit severe you apparently need to go to ski country or the Cape.
-
Nice warm day. Dews mixed out nicely over the interior. It's really not that bad out at all...pretty much U80s here. 86.1F max on the Davis.
-
Overwatered maybe?